Full study title: Monitoring oEdema in Heart Failure to Improve Function and Reduce Hospitalisation Risk (ME-HF).
Helping people with heart failure stay well at home and avoid hospital visits.
The ME-HF study is a UK-wide clinical trial looking at whether a new, simple home device can help spot early signs of fluid build-up (oedema) in people living with heart failure. The study is run across NHS hospitals and GP practices and is funded by the National Institute for Health and Care Research (NIHR).
Why are we doing this study?
Many people with heart failure carry extra fluid in their legs and ankles. This can be a sign that the heart is under strain.
Often, this fluid builds up days or even weeks before someone feels unwell. If we could spot these changes earlier, we may be able to adjust treatment sooner and prevent a hospital visit.
At the moment, people are advised to weigh themselves daily, but this can be difficult to remember or manage, especially when living with several health conditions.
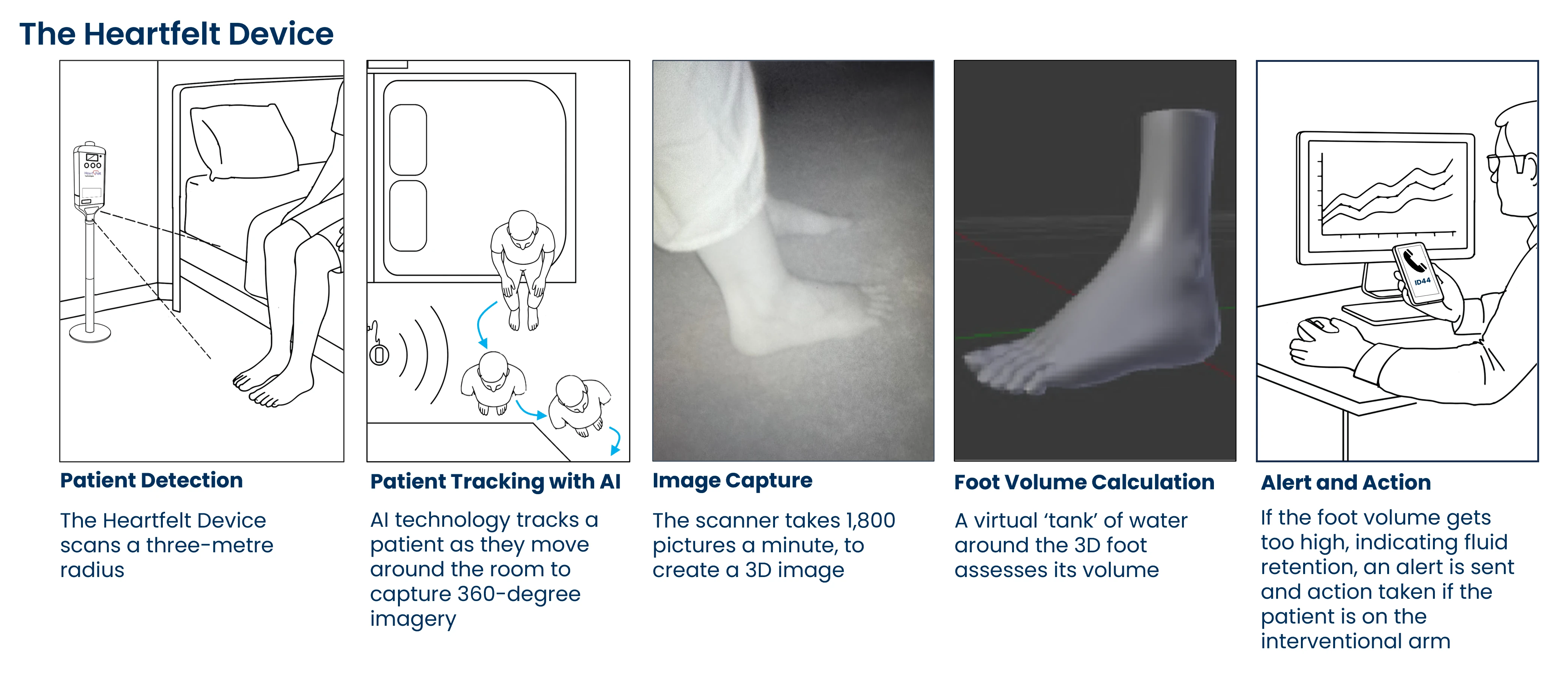
What is the Heartfelt Device?
The Heartfelt device is a small, non-invasive home based sensor that measures the volume of your feet and lower legs as you walk past it.
- There is no need to touch the device, stand on it, press any buttons, or wear anything.
- It simply works in the background while you get on with your day.
- The device securely sends measurements to the study team.
If the device detects that your leg swelling is increasing, it can send a health alert to you and the clinical team looking after you. This may lead to a phone call or review to check symptoms and, if needed, adjust treatment.
What does the study involve for participants?
To understand whether the Heartfelt device truly helps reduce hospital visits and improve quality of life, we need to compare it against usual care. This means that some participants will receive alerts from the device, while others will not. The group without alerts acts as a control group, which is the gold-standard way to test whether a new device works. This is the type of evidence that organisations like NICE and the NHS require before considering wider adoption.
Standard Care Group
You continue with your usual heart failure care and are encouraged to weigh yourself daily. A Heartfelt device will still be installed in your home and will take measurements in the background, but no alerts will be sent to you or your clinical team.
Heartfelt Group
You receive the same standard care, plus the Heartfelt device will monitor your foot and leg volume automatically each day. If the device detects a concerning change, alerts will be sent to you and to your clinical team so that they can review your symptoms and consider early treatment if needed.
Everyone in the study:
- Has the device installed at home (in both groups, so no one knows which group they are in).
- Completes short check-ins at 3, 6, 9, and 12 months. This is done over the phone, email or post, you decide what is most convenient to you.
- Any clinical events (such as hospital visits) are tracked from NHS records.
The study lasts 12 months for each participant.
Who can take part?
People who:
- Are aged 18 or over
- Have a diagnosis of heart failure
- Have had leg swelling in the last year
- Are taking a loop diuretic (such as furosemide)
- Live at home in the UK in a place where the device can be installed
Your local clinical team can confirm full eligibility.
What are we trying to find out?
We want to know whether using the Heartfelt device, alongside usual NHS care:
- Reduces unplanned hospital visits
- Helps people feel better and more in control of their condition
- Supports earlier treatment decisions
- Is practical and acceptable for patients, carers, and clinicians
- Offers good value for the NHS
Is the device safe?
Yes. The device is non-contact, uses a safe imaging method, and has been used in previous NHS studies. It does not replace usual care. If you feel unwell, you should always seek help as normal. It has a CE mark, which means that it meets the stringent criteria for medical devices to be placed on the UK and European markets.
Your privacy is protected: the device only captures images of your lower legs, and no personal details are stored with these images.
Who is running the study?
The study is sponsored by Heartfelt Technologies Ltd, led by researchers at Manchester University NHS Foundation Trust and The University of Glasgow, and delivered in partnership with at least 15 NHS sites across the UK.
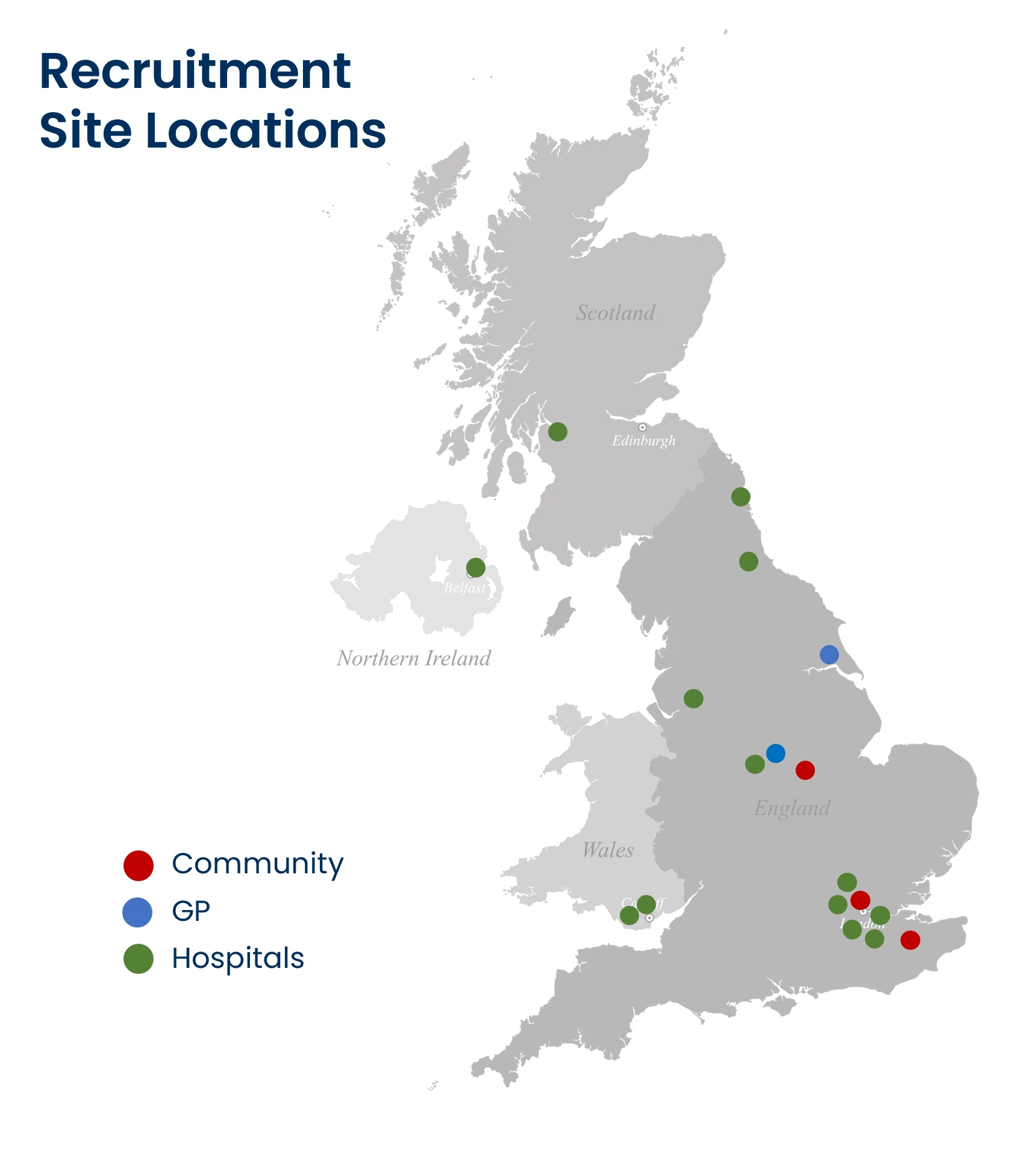
Where is the study taking place?
Hospitals and GP practices across the UK are taking part. You can see on the map below the sites which are planning to start recruitment shortly.